07 Jun 2023 - A cytosolic surveillance mechanism orchestrates mitochondria-to-nucleus communication
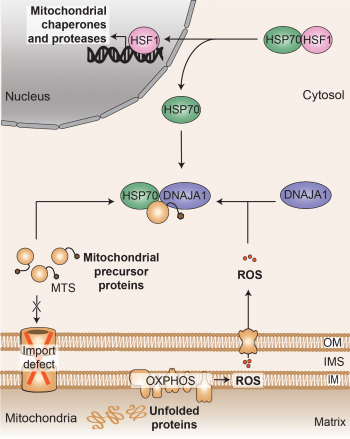
Mitochondria play a crucial role in various cellular functions, necessitating the maintenance of proper protein functionality through protein quality control pathways. When mitochondria experience protein misfolding stress, they initiate a transcriptional response to enhance the expression of mitochondrial chaperones and proteases. This process, known as the mitochondrial unfolded protein response (UPRmt), aims to restore proteostasis within the mitochondria. Although the UPRmt was discovered more than two decades ago, the exact signaling pathway by which mitochondria communicate with the nucleus to activate the UPRmt in humans has remained elusive.
A research team led by Christian Münch at the Institute of Biochemistry II (IBC2) has recently uncovered a cytosolic surveillance mechanism that detects and activates the UPRmt in humans. This pathway is triggered by two signals originating from mitochondria: reactive oxygen species (ROS) and precursor proteins accumulating in the cytosol. These signals converge at the DNAJA1-HSF1 axis, resulting in the direct initiation of the transcriptional response involving mitochondrial chaperones and proteases. The team's findings, published in Nature, unveil a novel connection between mitochondrial and cytosolic proteostasis, providing valuable insights into diseases associated with inter-organelle protein quality control pathways, including aging.
F.X. Reymond Sutandy, Ines Gößner, Georg Tascher, Christian Münch.